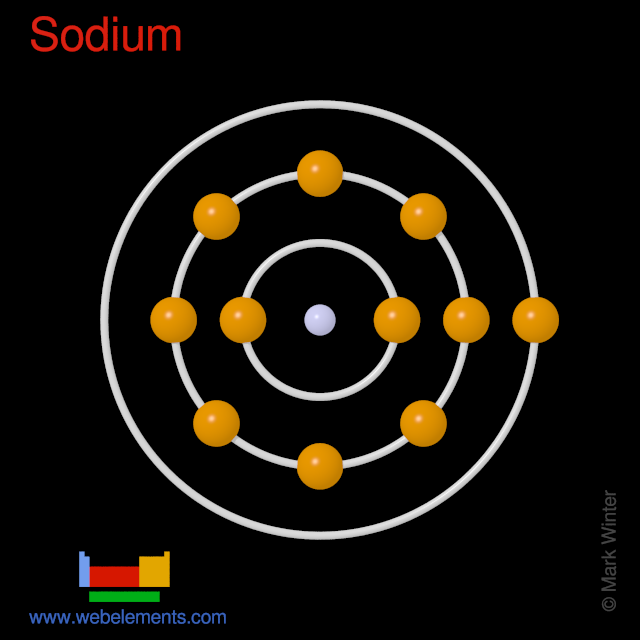
Na Atom
What is the ground-state electron configuration of a neutral atom of sodium?

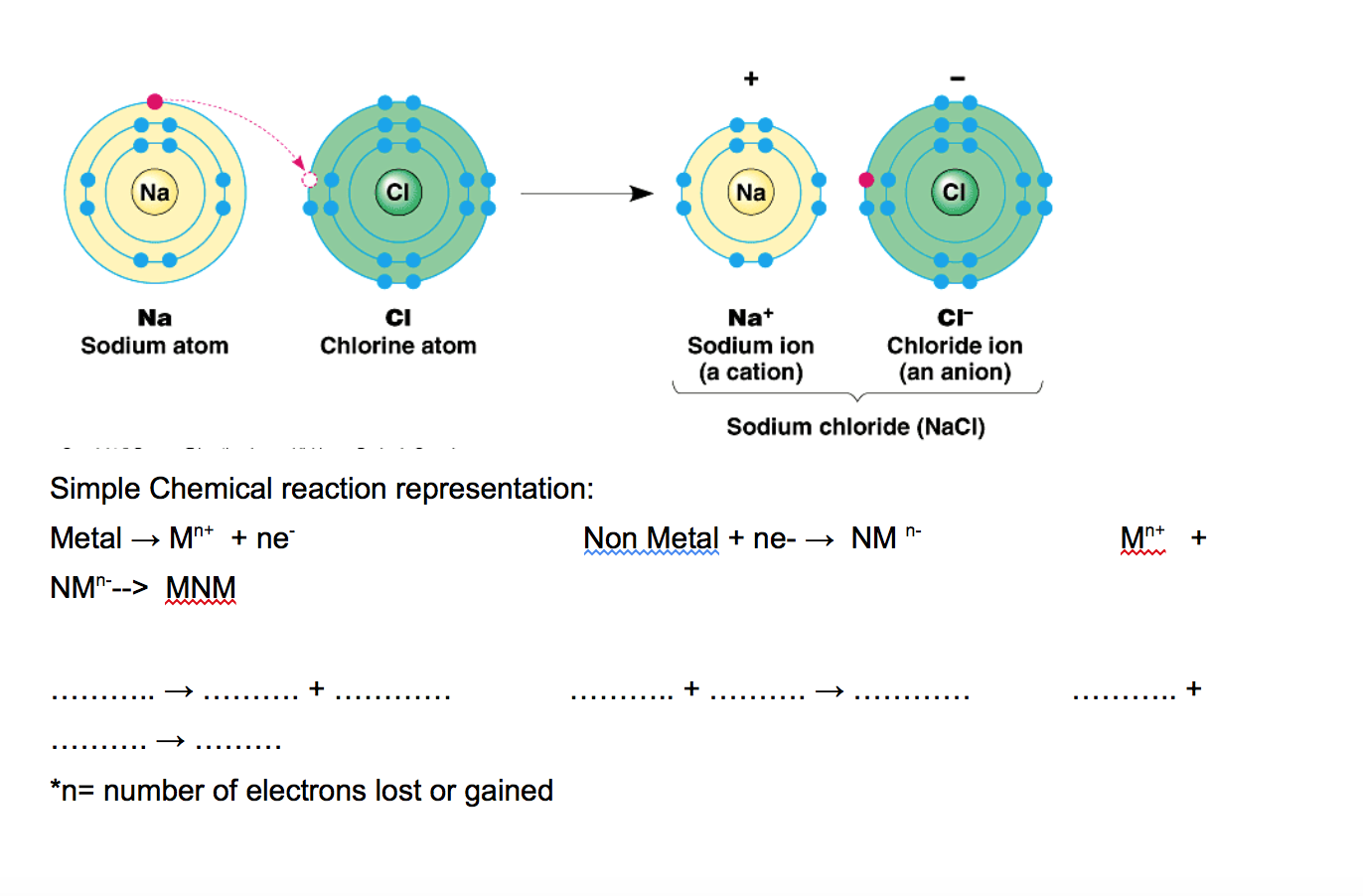
Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Sample Learning Goals Use the number of protons, neutrons, and electrons to draw a model of the atom, identify the element, and determine the mass and charge. Predict how addition or subtraction of a. Symbol Origin:From the Latin word natrium(sodium). As we begin our exploration of the third period (row) of the periodic table, we find the element sodium (Na). Being in the first column, sodium is a member of the alkali metal family with potassium (K) and lithium (Li). Sodium's big claim to fame is that it's one of two elements in your table salt. When you look at a periodic table of the elements, you see that sodium has an atomic weight of 23. This means that a single atom of sodium weighs 23 atomic mass units (AMUs), but it also means that. Na is a neutral form of the sodium atom. Na+ on the other hand has a positive charge of 1. This + means that the atom has lost 1 electron, making it's charge more positive that it was before. The sodium atom loses its outer electron to become a sodium ion. But now only 10 electrons (10 negative charges).
1 Answer
Na Atomic Mass
Explanation:
And so in the neutral atom, we gots ELEVEN electrons to distribute...
And, as is typical, this electronic configuration reflects Periodic Structure....sodium is an
Sodium metal is thus a good reducing agent that readily forms
Related topic
Related questions
››Convert mole to atom
Please enable Javascript to usethe unit converter.
Note you can turn off most ads here:
https://www.convertunits.com/contact/remove-some-ads.php
››More information from the unit converter
How many moles in 1 atom?The answer is 1.660538863127E-24.
We assume you are converting between mole and atom.
You can view more details on each measurement unit:
moles oratom
The SI base unit for amount of substance is the mole.
1 mole is equal to 6.0221415E+23 atom.
Note that rounding errors may occur, so always check the results.
Use this page to learn how to convert between moles and atoms.
Type in your own numbers in the form to convert the units!
››Quick conversion chart of moles to atom
1 moles to atom = 6.0221415E+23 atom
2 moles to atom = 1.2044283E+24 atom
Na Atom Size
3 moles to atom = 1.80664245E+24 atom

4 moles to atom = 2.4088566E+24 atom
5 moles to atom = 3.01107075E+24 atom
6 moles to atom = 3.6132849E+24 atom
7 moles to atom = 4.21549905E+24 atom
8 moles to atom = 4.8177132E+24 atom
9 moles to atom = 5.41992735E+24 atom
10 moles to atom = 6.0221415E+24 atom
Na Atom Size
››Want other units?
You can do the reverse unit conversion fromatom to moles, or enter any two units below:
››Common amount of substance conversions
moles to centimol
moles to decimol
moles to micromol
moles to nanomol
moles to millimol
moles to picomol
moles to kilomol
moles to molecule
››Definition: Mole
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol.'
››Definition: Atom
This site uses an exact value of 6.0221415 x 1023 for Avogadro's number. This is the number of atoms in 1 mole of a chemical element.
Na Atomic Symbol
››Metric conversions and more
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!
